
10 moles of HCL is added to excess to magnesium and forms 4 moles of hydrogen gas then Percentage - Brainly.in

Effect of HCl (0.1 to 2 mol/L) concentration on transport of As(III)... | Download Scientific Diagram
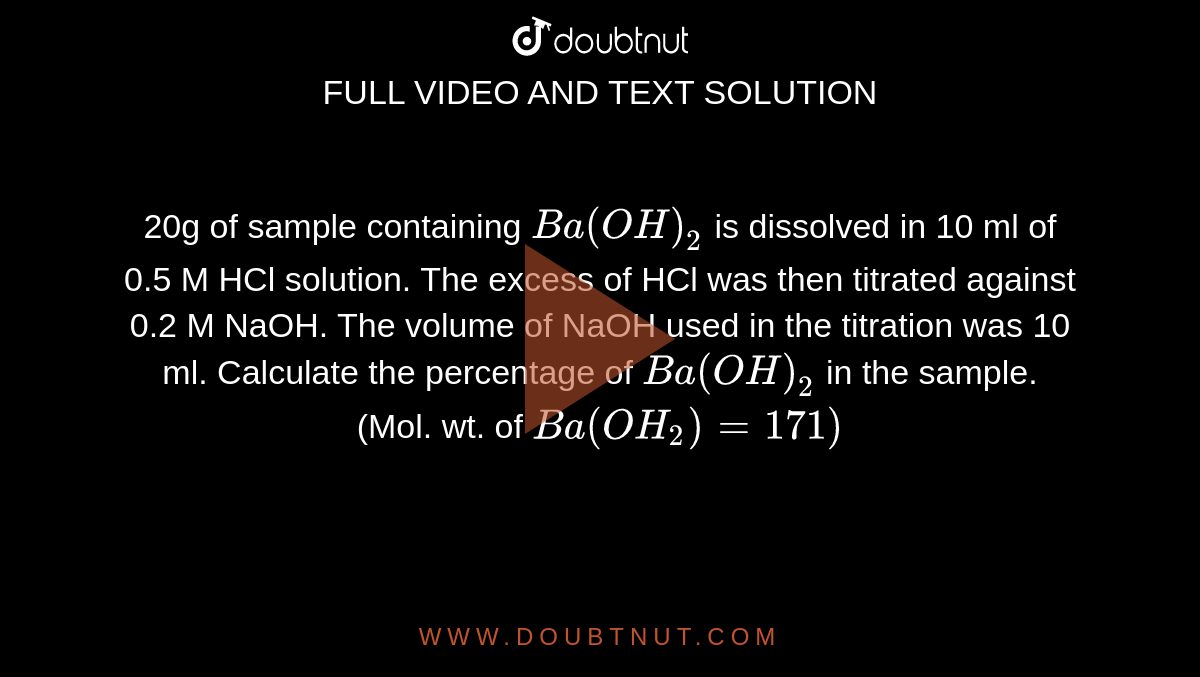
20g of sample containing Ba(OH)(2) is dissolved in 10 ml of 0.5 M HCl solution. The excess of HCl was then titrated against 0.2 M NaOH. The volume of NaOH used in

Hydrochloric Acid, c(HCl)=0.1 mol/L (0.1N) Titripur , MilliporeSigma, Quantity: 1 L | Fisher Scientific
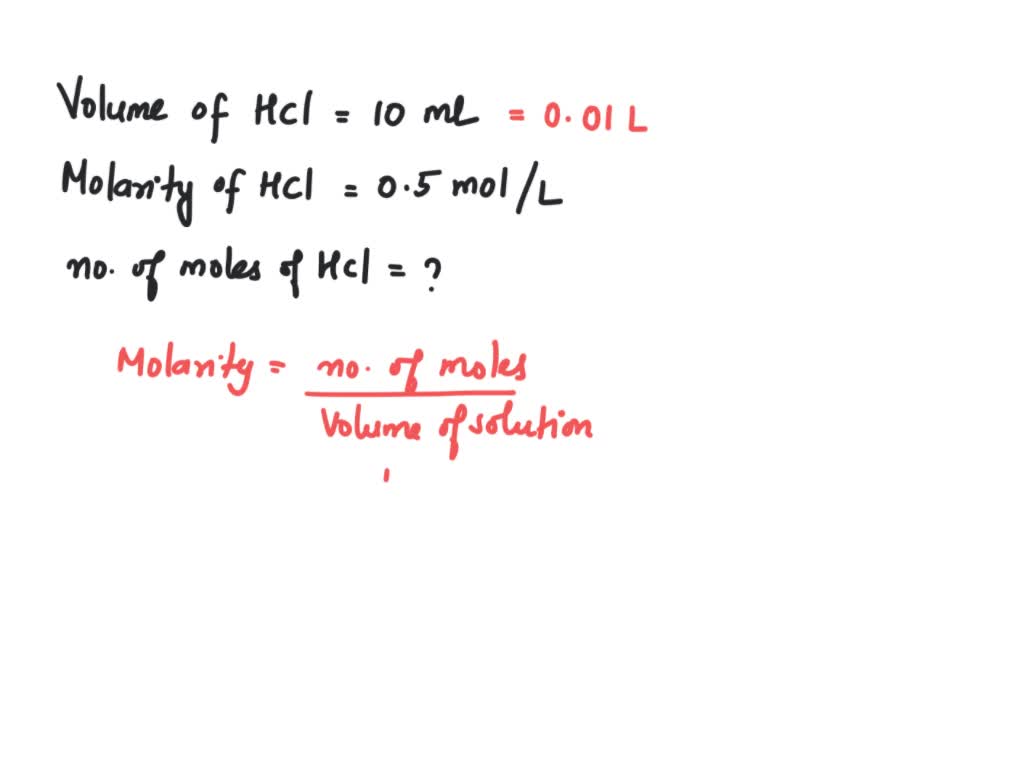
SOLVED: 1 How many moles of HCl are there in 10 mL of a solution with a concentration of 0.5 molL-I? swered Select one: 5 mole 0.05 mole of 200 Jestion 50 mole 0.5 mole












