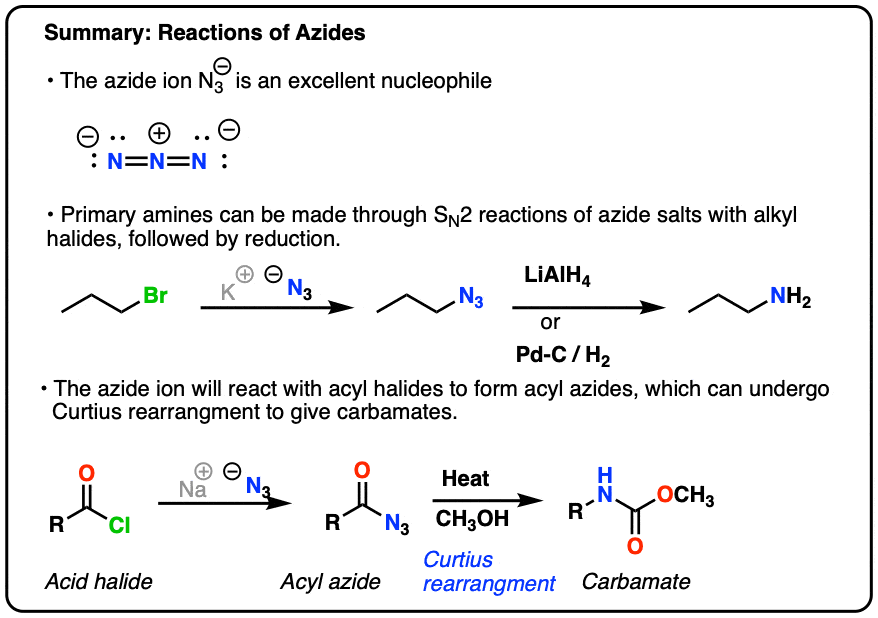
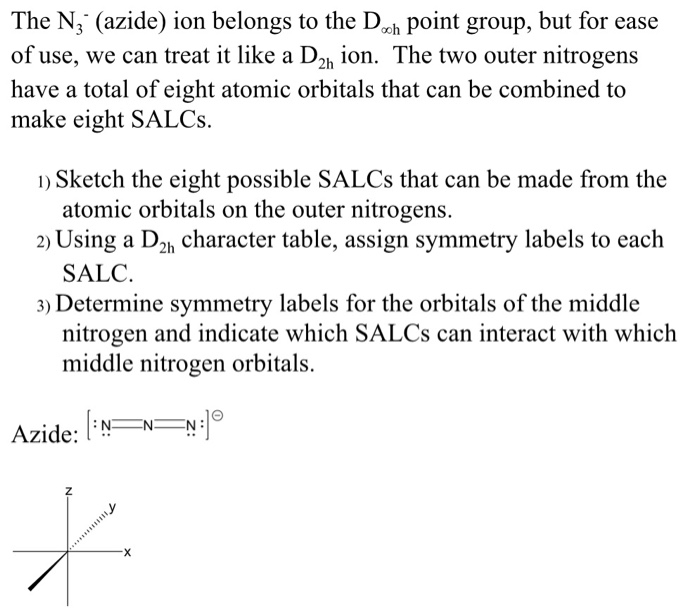
Draw and explain the Lewis structure for the azide ion, showing all its possible resonance structures. Give the name of the molecular geometry and the hybridization around the central atom. | Homework.Study.com

The azide ion (N_3) is a linear triatomic molecule. Determine the bond order and comment on how it compares to the Lewis Dot diagram for this molecule. | Homework.Study.com
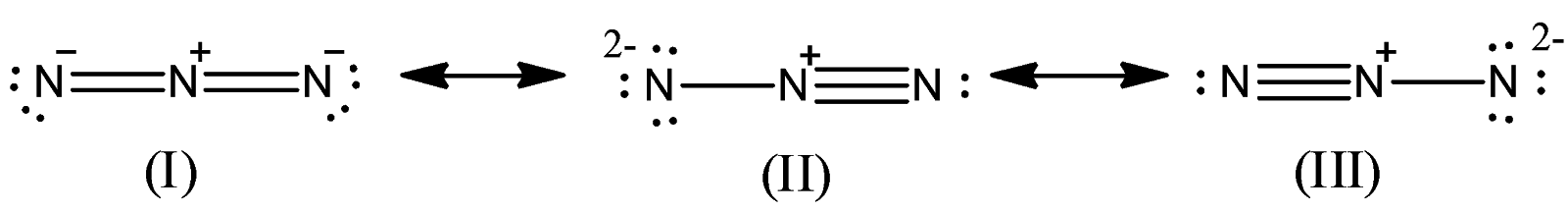
Azide ion (${{N}_{3}}^{-}$) exhibits an N-N bond order of 2 and may be represented by resonance structures I, II and III given below. Select correct statements.\n \n \n \n \n (A) Structures

Draw and explain the Lewis structure for the azide ion, showing all its possible resonance structures. Give the name of the molecular geometry and the hybridization around the central atom. | Homework.Study.com

Draw resonance formulas for the azide ion, and for the nitronium ion. Decide which resonance formula is the best description of each ion. | Homework.Study.com
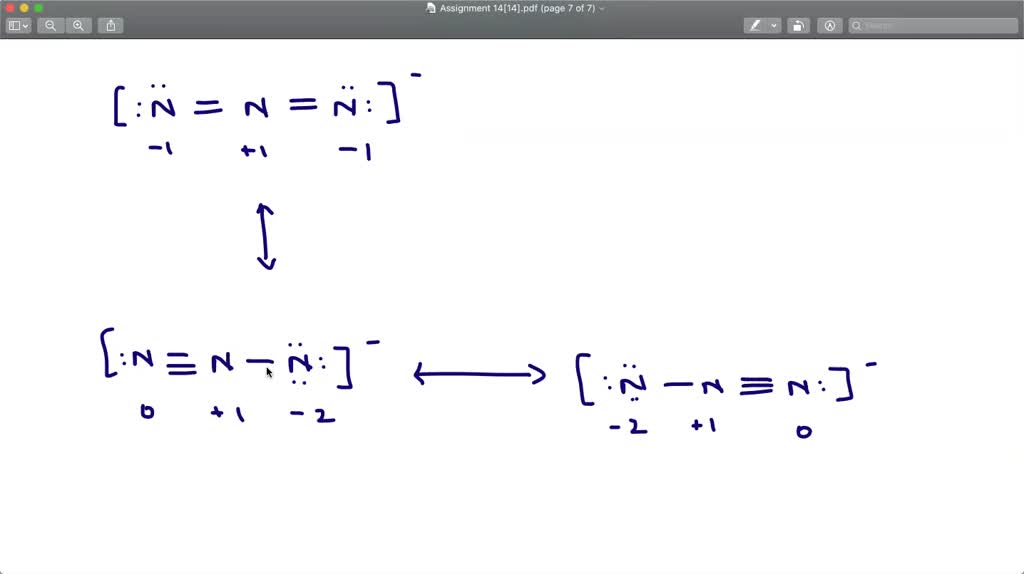
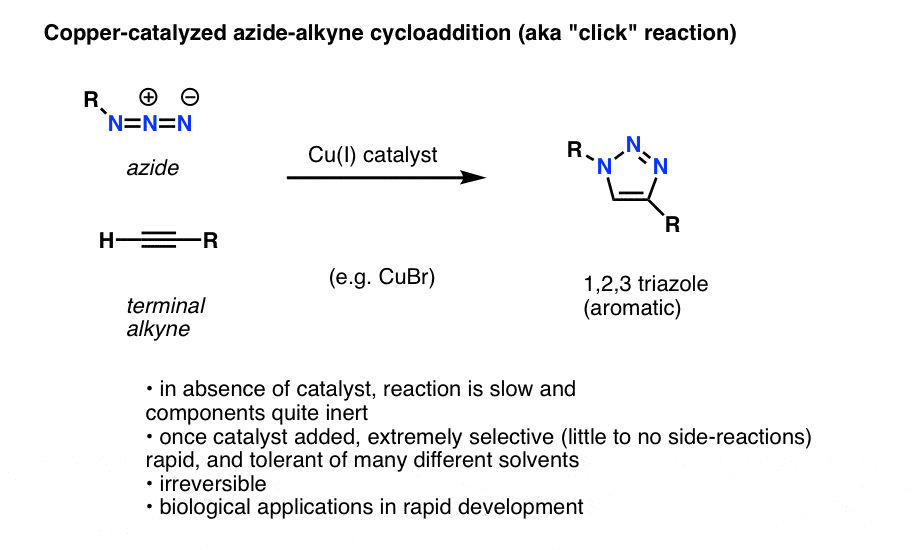



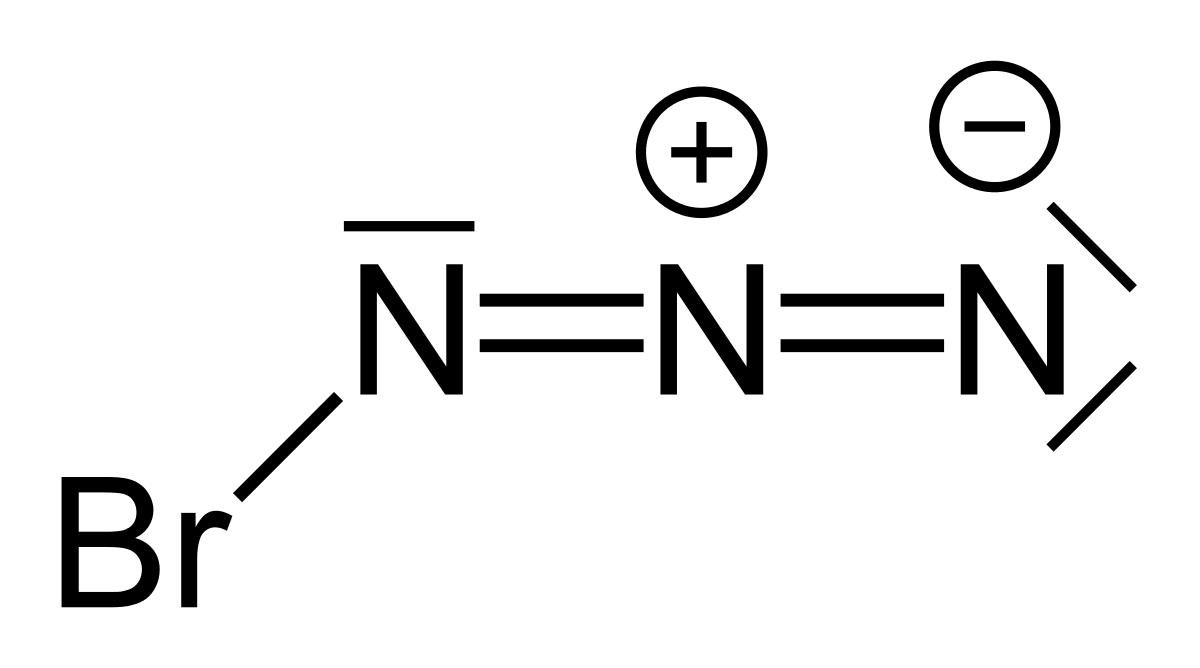

![Azides [N3(–)] - ChemistryScore Azides [N3(–)] - ChemistryScore](https://chemistryscore.com/wp-content/uploads/2019/11/Azides-N33-1024x206.png)