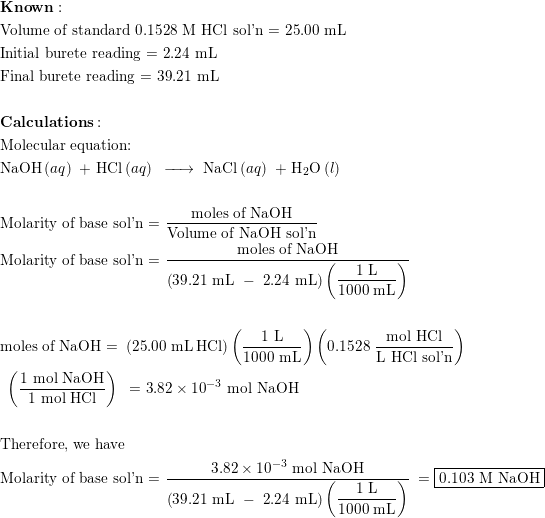
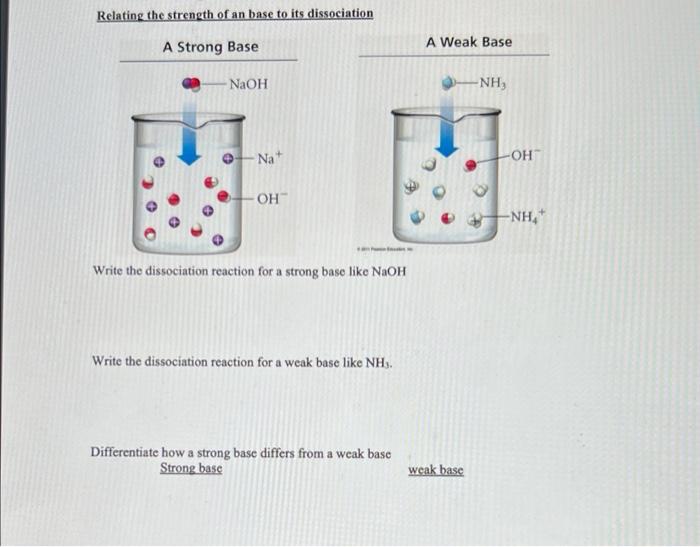
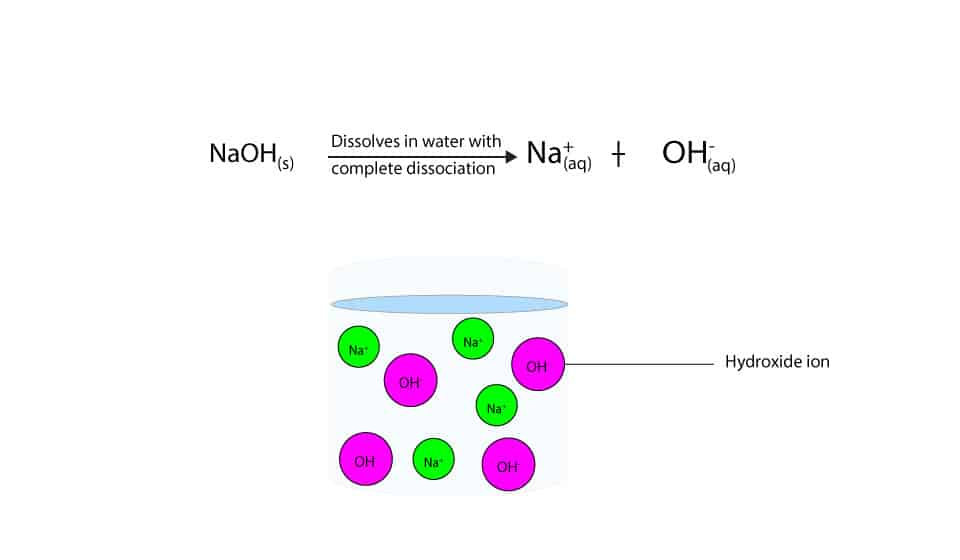
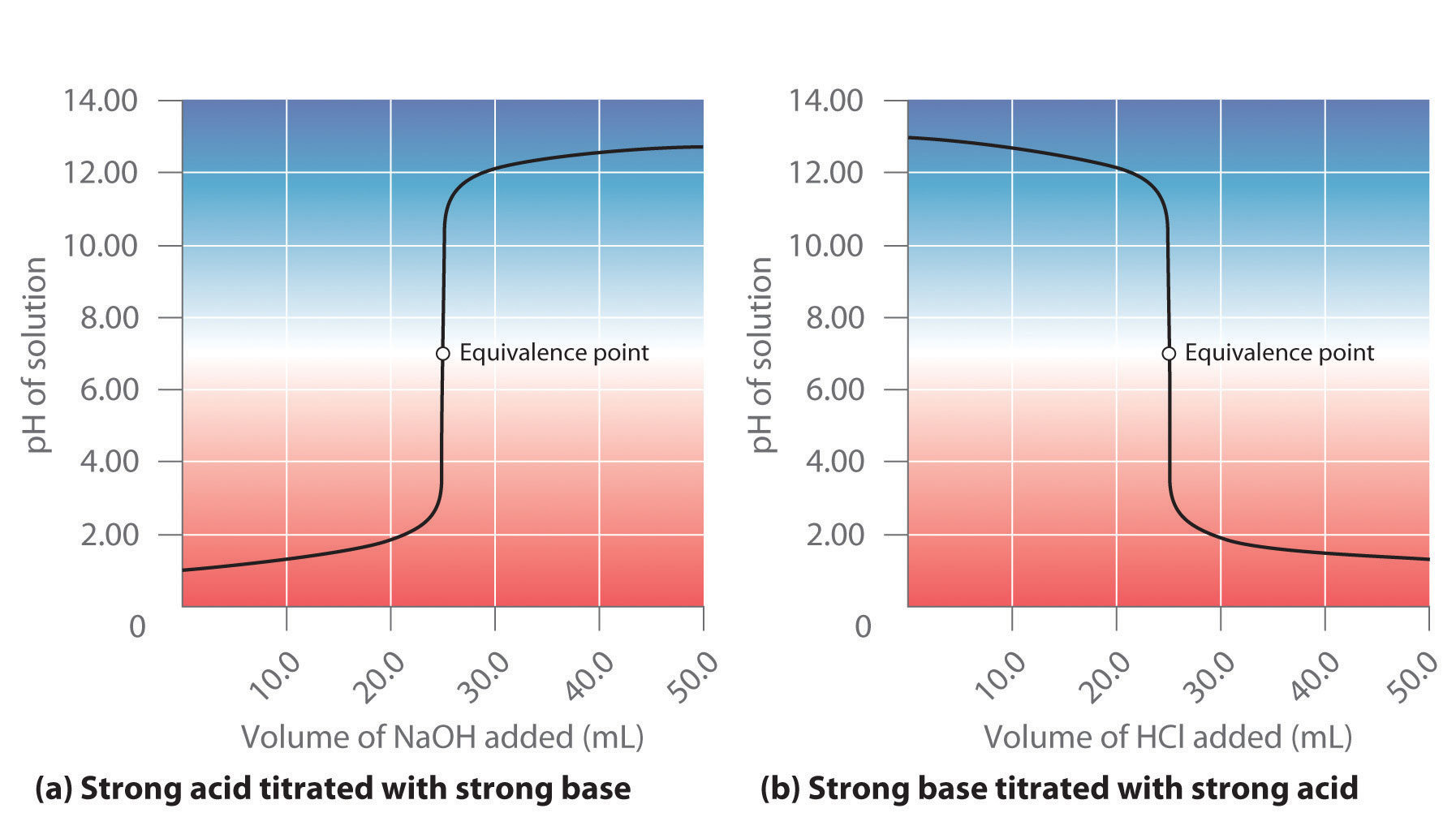
Effect of base (NaOH) and acid (HCl) additions on changes in buffering... | Download Scientific Diagram
Sodium hydroxide, caustic soda, lye molecule. NaOH is highly caustic base and alkali, ionic compound. Structural chemical formula and molecule model Stock Vector Image & Art - Alamy
Sodium hydroxide, caustic soda, lye molecule. NaOH is highly caustic base and alkali, ionic compound. Structural chemical formula and molecule model Stock Vector Image & Art - Alamy

Acid – Base Reaction. Chemical Reaction Neutralization The Acid And Base Properties, Producing A Salt And Water.
Acids and bases, acid-base reaction, neutralization reaction, HCl, NaOH, NaCl, H2O, salt, water, chemical reaction, test setup, erlenmeyer, laboratory materials Stock ベクター | Adobe Stock

-in-water-01.jpg)