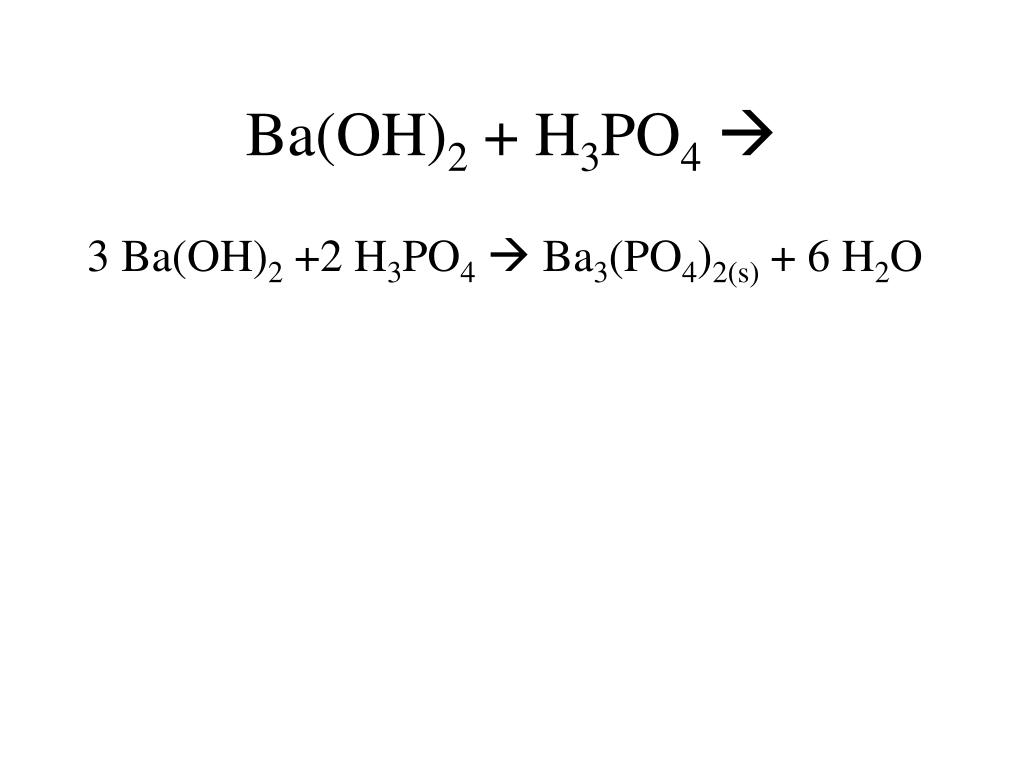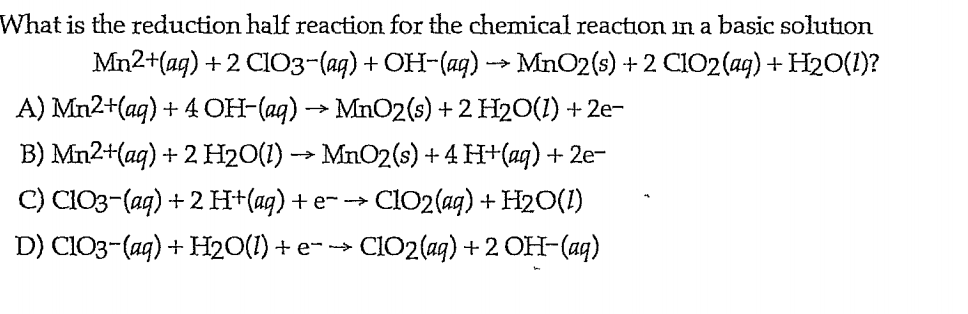Calculate the volume of 0.025 M Ca(OH)_2 solution which can neutralise 100 mL of 0.0001 M H_3PO_4. | Homework.Study.com
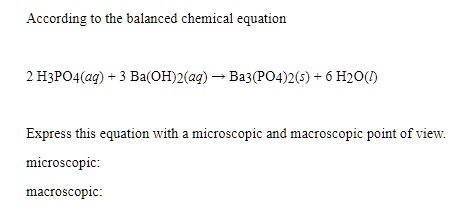
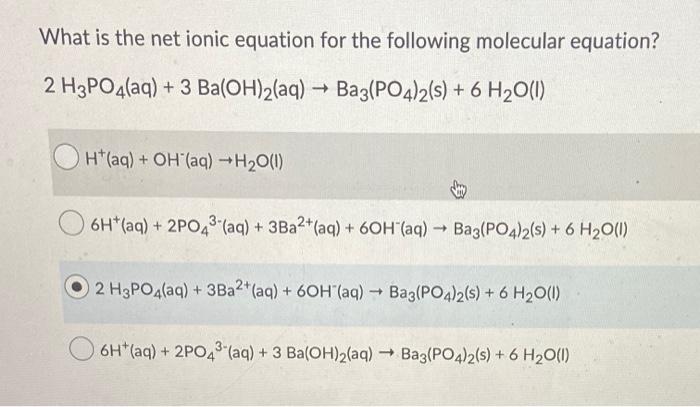
SOLVED: According to the balanced chemical equation 2 H3PO4(aq) Ba(OH) (aq) Ba3(PO4)2(5) Hzo() Express this equation With microscopic and macroscopic point of view: micfoscopic: macroscopic:
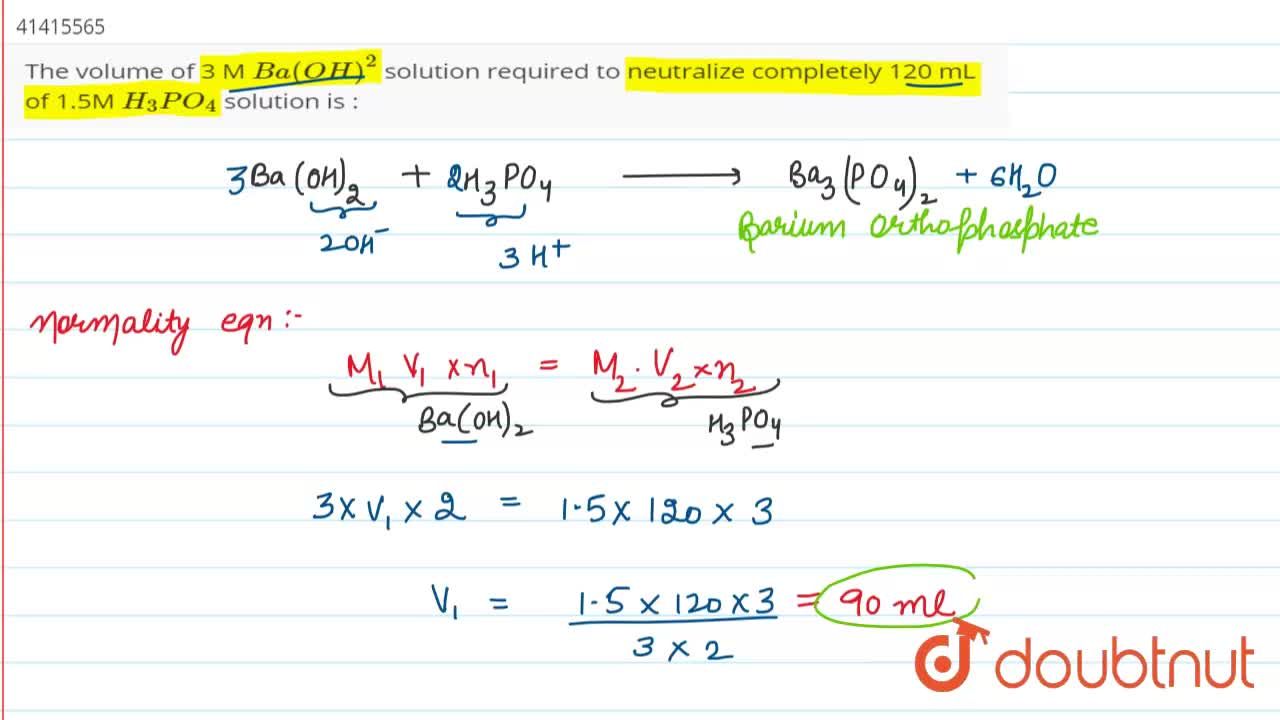
The volume of 3 M Ba(OH)^(2) solution required to neutralize completely 120 mL of 1.5M H(3)PO(4) solution is :

SOLVED: Barium hydroxide and phosphoric acid react as follows: 3 Ba(OH)2(s) + 2 H3PO4(aq) –> Ba3(PO4)2(aq) + 6 H2O(l) If 39.5 g of Ba(OH)2 are allowed to react with 51.0 g of

OneClass: 8) the equations below, write the products for each reaction, and balance the For equations...

OneClass: Barium hydroxide is a strong base and can be used to titrate acids. The (unbalanced) reacti...